Why is the Coronavirus vaccine taking so long to deploy? As Boris blames regulators for a sluggish start, fingers are also pointed at bureacratic hurdles for volunteers and a shortage of vials
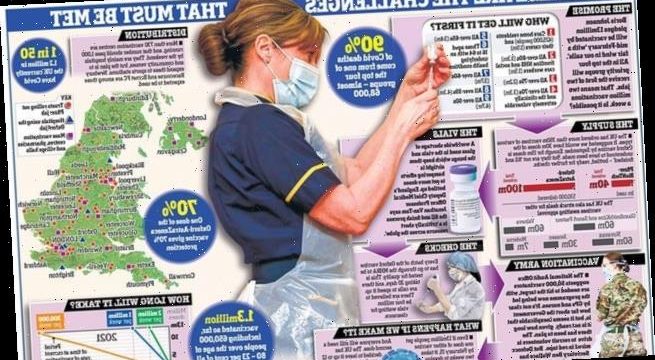
- Worldwide demand for glass vials is just one factor affecting vaccine rollouts
- A special kind of glass is required to keep the vaccine inside stable
- Only a handful of companies make the vials, including Schott in Germany
Britain has vaccinated 1.3million people in just under a month… but the target is two million a week.
That’s the rate needed to protect the four most vulnerable groups by February 15 – including everyone over 70.
Boris Johnson has blamed regulators for the sluggish start, warning that their strict protocols have limited how quickly the vaccine programme can be accelerated.
The Business Secretary, Alok Sharma, had promised in May that 30million doses of the vaccine from Oxford and AstraZeneca would be ready by September. Now, four months on from that deadline, our stocks are still falling short of the target two million a week.
The Prime Minister said of the inoculation drive: ‘The rate limiting the factor at the moment is making sure that we can get enough vaccine where we want it fast enough.
One of the problems as you know is that the AstraZeneca vaccine needs to be properly batch-tested, properly approved before it can be put into people’s arms and this is just a process that takes time to do… but we will be ratcheting it up over the next days and weeks ahead.’
People queue outside a Covid-19 vaccination centre in Guy’s hopsital in London on Tuesday
Other serious difficulties include worldwide demand for glass vials. In addition, those hoping to join an army of volunteers to boost the national effort have been tangled up in reams of red tape.
Ministers insist that the NHS has the capacity to deliver two million doses a week – once it receives supplies from manufacturers which have been checked by regulators. The Medicines and Healthcare products Regulatory Agency (MHRA) insists it is capable of batch testing – but has been waiting to receive more doses from manufacturers.
Chief medical officer Chris Whitty told yesterday’s Downing Street press conference that the six-week target was ‘realistic but not easy’. So, as Britain embarks upon the biggest vaccination drive in its history, what are the main hurdles?
Is batch testing too slow?
Use London Nightingale? What an ExCellent idea!
London’s Nightingale hospital is to double up as a mass vaccination hub, providing jabs seven days a week from 8am to 8pm.
Work continued last night at the ExCel, Europe’s biggest conference centre. With cases soaring in the capital, the Docklands site has already been ‘reactivated’ to ensure it can relieve pressure on the NHS if necessary.
Epsom racecourse is among several other landmarks set to offer jabs as Britain’s vaccination programme gathers pace.
Vaccination drive: Moving barriers at the ExCel Centre
Groundwork: Tents on the west side of the Docklands site
Each batch must be tested for quality by the National Institute for Biological Standards and Control (NIBSC), part of the MHRA. The process can take up to 20 days.
A sample from each vaccine batch – which can contain hundreds of thousands of doses – is biologically tested for quality and safety.
Manufacturers must also carry out their own tests on each batch before submitting results as evidence to the NIBSC. Delays in providing these details – or any failure to meet standards – can slow the whole process down.
Only once both sets of tests have been completed – and the manufacturer’s results deemed acceptable – is a batch released by regulators for use by the NHS.
More doses are now being produced, which increases the workload for laboratories handling quality control. An MHRA spokesperson said: ‘We are working closely with the [Oxford vaccine] manufacturer, AstraZeneca, to ensure that batches of the vaccine are released as quickly as possible.
‘NIBSC has scaled-up its capacity to ensure that multiple batches can be tested simultaneously, and that this can be done as quickly as possible, without compromising quality and safety.’
Some observers have pointed out that the MHRA managed to speed up the process which saw Covid vaccines cleared for use. Critics might wonder why the same can’t be done at this stage, too.
Are there enough vials?
Drugs firms warned about a potential shortage of vials as far back as May, given the massive worldwide demand for vaccines.
The tubes are made from borosilicate glass, which keeps vaccines in the requisite stable state during storage and transportation. The glass is chemically inert, meaning there is no interaction between the container and the liquid inside it. This is crucial, as any chemical interference could affect the vaccine. Only a handful of companies make the vials, with Schott in Germany one of the leading producers.
Industry insiders have suggested that the UK needs to ramp up production itself to stop its reliance on overseas companies. Dave Dalton, chief executive of the trade body British Glass, said the supply chain ‘needs to be strengthened and improved’, adding that the supply of medical glass and vials was something that the industry had raised itself – and is ready to help sort out.
Advanced nurse practitioner Justine Williams (left) prepares to administer a dose of the AstraZeneca/Oxford Covid-19 vaccine to 82-year-old James Shaw, the first person in Scotland to receive the vaccination
Jonathan Van-Tam, England’s deputy chief medical officer, suggested issues with so-called ‘fill and finish’ materials, including glass vials, could hinder the vaccine’s rollout. ‘The only thing that is going to slow us down is batches of vaccines becoming available,’ he said during a Downing Street briefing. ‘Many of you know already that it’s not just about vaccine manufacture. It’s about fill and finish, which is a critically short resource across the globe.’ The Department of Health denies there are any vial shortages.
The UK has manufactured around 15million doses of the Oxford-Astra-Zeneca vaccine so far, with plants in Germany and the Netherlands providing more of the early batches. However, only four million doses have been through the fill and finish process – and are still awaiting MHRA clearance.
Do we have enough people to give jabs?
Retired doctors have complained that red tape has stopped them from returning to the frontline to deliver Covid vaccines.
Health Secretary Matt Hancock vowed to tackle the problem, with some would-be volunteers asked for 21 documents proving they are trained in areas such as counter- terrorism and racial equality.
NHS England says it has ‘tens of thousands’ of vaccinators ready to be called upon when more doses are ready to be administered. The army includes healthcare workers such as physiotherapists, nurse practitioners and paramedics who were given the green light to administer jabs after a rule change this summer.
An NHS spokesman said there were ‘thousands more’ in training, but added that the Health Service was confident it has enough people to staff the vaccination programme as it expands. Medics from the Armed Forces are also set to be deployed.
Practice Sister Tina Sutton (left) administers a dose of the AstraZeneca/Oxford Covid-19 vaccine to Derek Davies Games at the Pontcae Medical Practice in Merthyr Tydfil in Wales
Are checks too long?
BUREAUCRACY has been blamed for slowing down the actual act of vaccination, too, with patients facing lengthy quizzes about their medical history.
Some say they underwent a 15-minute medical questionnaire over the phone before being asked for many of the same details when they arrived for their jab.
Once vaccinated, patients should be monitored for 15 minutes to ensure they have no adverse reactions – meaning the whole process can take around 45 minutes. Doctors have suggested that this should be streamlined, as it severely limits how many vaccines can be delivered at any given site in one day.
What about the manufacturers?
Pharmaceutical companies have hit back at any suggestion that they are to blame for delays.
Pfizer and BioNTech – producers of the first vaccine approved by the MHRA – said they have now sent ‘millions’ of doses to the UK, with up to 40million expected in the coming months.
AstraZeneca has confirmed it expects to be able to supply two million doses of the Oxford vaccine to the NHS every week by the second half of this month, with at least 20million due by the end of March. The jab has only been available at hospital hubs so far – but GP surgeries will join the rollout tomorrow.
Source: Read Full Article